
Chemistry, 22.10.2020 21:01, evanwall91
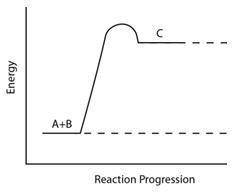
Consider the reaction pathway graph below.
Which statement accurately describes this graph?
A) It represents an endothermic reaction because the product has more energy than the reactants.
B) It represents an exothermic reaction because the product has more energy than the reactants.
C) It represents an endothermic reaction because the reactants have more energy than the product.
D) It represents an exothermic reaction because the reactants have more energy than the product.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Do you know the correct answer?
Consider the reaction pathway graph below.
Which statement accurately describes this graph?
A...
A...
Questions in other subjects:


Mathematics, 30.10.2020 07:20

Mathematics, 30.10.2020 07:20

Social Studies, 30.10.2020 07:20


Mathematics, 30.10.2020 07:20

Biology, 30.10.2020 07:20


Mathematics, 30.10.2020 07:20

Mathematics, 30.10.2020 07:20







