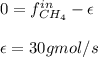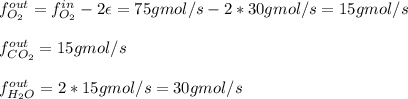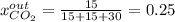Chemistry, 21.10.2020 16:01, HaileyAnn04
For the combustion of methane presented in Example 5.4, the chemical reaction is CH4 +2O2 →CO2 +2H2O Suppose that methane flows into a burner at 30 gmol/s, while oxygen flows into the same burner at 75 gmol/s. If all the meth- ane is burned and a single output stream leaves the burner, what is the mole fraction of CO2 in that output stream? Hint 1: Does the fact that all the methane is burned mean that all the oxygen is burned also? Hint 2: Find the molar flow rate of each component gas in the outlet gas ("flue gas").

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, audrey1256
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 21:00, nsutton9985
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1
Do you know the correct answer?
For the combustion of methane presented in Example 5.4, the chemical reaction is CH4 +2O2 →CO2 +2H2O...
Questions in other subjects:

Mathematics, 09.03.2021 03:30

Mathematics, 09.03.2021 03:30


Social Studies, 09.03.2021 03:30


Mathematics, 09.03.2021 03:30


Mathematics, 09.03.2021 03:30


Chemistry, 09.03.2021 03:30


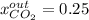
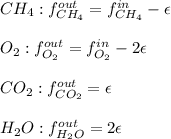
 accounts for the reaction extent. However, as all the methane is consumed, from the methane balance:
accounts for the reaction extent. However, as all the methane is consumed, from the methane balance: