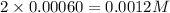Hydrogen sulfide decomposes according to the following reaction, for which Kc=9.30E-8 at 700 degrees Celsius. 2 H2S(g) --> 2 H2(g) + S2(g) If 0.29 moles of H2S is placed in a 3.0-L container, What is the equilibrium concentration of H2(g) at 700 degrees Celsius?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 03:00, actheorian8142
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3


Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
Do you know the correct answer?
Hydrogen sulfide decomposes according to the following reaction, for which Kc=9.30E-8 at 700 degrees...
Questions in other subjects:

Health, 23.12.2020 21:20


Geography, 23.12.2020 21:20



Mathematics, 23.12.2020 21:20

Mathematics, 23.12.2020 21:20




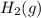 at 700 degrees Celsius is 0.0012 M
at 700 degrees Celsius is 0.0012 M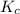
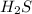 = 0.29 mole
= 0.29 mole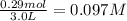
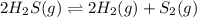
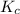 is written as:
is written as:![K_c=\frac{[H_2]^2\times [S_2]}{[H_2S]^2}](/tpl/images/0827/9052/13e21.png)
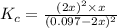
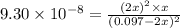
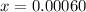
![[H_2]](/tpl/images/0827/9052/08a38.png) = 2x=
= 2x=