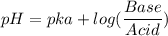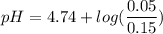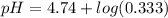Chemistry, 21.10.2020 16:01, anonymous115296
Calculate the expected pH of the buffer after the addition of 1.0 mL of 1M HCl. Remember that you are using 50 mL of the buffer, so be sure to calculate the moles of acetic acid and acetate in 50 mL of the buffer.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, KindaSmartPersonn
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 23:40, tilievaughn14
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
Do you know the correct answer?
Calculate the expected pH of the buffer after the addition of 1.0 mL of 1M HCl. Remember that you ar...
Questions in other subjects:






History, 06.05.2020 18:02


Mathematics, 06.05.2020 18:02


English, 06.05.2020 18:02