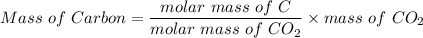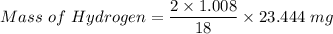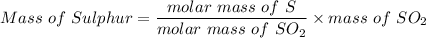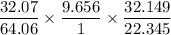Chemistry, 21.10.2020 16:01, PONBallfordM89
A 32.149 mg sample of a chemical known to contain only carbon hydrogen sulfur and oxygen is put into a combustion analysis apparatus yielding 57.271 mg of co2 and 23.444 mg of h2o. In another experiment 22.345 mg of the compound is reacted with excess oxygen to produce 9.656 mg of sulfur dioxide

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1


Do you know the correct answer?
A 32.149 mg sample of a chemical known to contain only carbon hydrogen sulfur and oxygen is put into...
Questions in other subjects:


Arts, 17.04.2021 01:00

History, 17.04.2021 01:00


World Languages, 17.04.2021 01:00

Mathematics, 17.04.2021 01:00

History, 17.04.2021 01:00

Chemistry, 17.04.2021 01:00

Mathematics, 17.04.2021 01:00

Mathematics, 17.04.2021 01:00