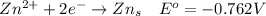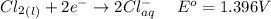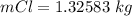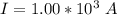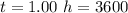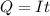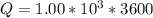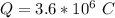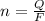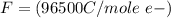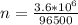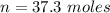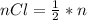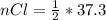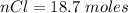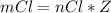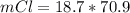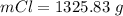Consider the rechargeable battery: Zn(s)0ZnCl (aq)7Cl2(aq)0Cl (l)0C(s) (a) Write reduction half-reactions for each electrode. From which electrode will electrons flow from the battery into a circuit if the electrode potentials are not too different from E 8 values

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 10:40, justicejesusfreak
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 17:40, Snowball080717
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
Do you know the correct answer?
Consider the rechargeable battery: Zn(s)0ZnCl (aq)7Cl2(aq)0Cl (l)0C(s) (a) Write reduction half-reac...
Questions in other subjects:

Chemistry, 21.10.2020 20:01


Health, 21.10.2020 20:01

Biology, 21.10.2020 20:01



Physics, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01


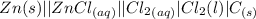
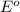 values
values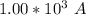 for 1.00 h , how many kg of
for 1.00 h , how many kg of 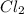 will be consumed
will be consumed