
Chemistry, 20.10.2020 01:01, aaronjin4443
PLz HELP ASAP! Mass of stoppered test tube plus metal 95 g
Mass of test tube and stopper 31.3g
Mass of calorimeter 2.5 g
Mass of calorimeter and water 46.1 g
Mass of water (subtraction) _ g
Mass of metal (subtraction) _ g
Initial temperature of water in calorimeter 24.0°C
Initial temperature of metal (assume 100°C
unless directed to do otherwise) 98.4°C
Equilibrium temperature of metal and water
in calorimeter 29.0°C
Δ 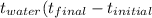 _ °C
_ °C
Δ 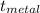 _ °C
_ °C
_ J
Specific heat of the metal _ J/g°C
Approximate molar mass of metal _ g/mol
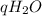

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, stellaglenn205
What reaction is taking place? 02 + c3h8 = h20 + co2
Answers: 1

Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Do you know the correct answer?
PLz HELP ASAP! Mass of stoppered test tube plus metal 95 g
Mass of test tube and stopper 31.3g
Questions in other subjects:





Mathematics, 21.04.2020 04:38


Spanish, 21.04.2020 04:39









