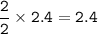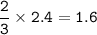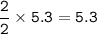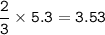Chemistry, 19.10.2020 04:01, mosesbrinker
23. For the reaction shown, calculate how many moles of each
product form when the given amount of each reactant com-
pletely reacts. Assume there is more than enough of the
other reactant.
2PbS(s) + 302(g) → 2PbO(s) + 2S02(8)
(a) 2.4 mol PbS
(b) 2.4 mol O2
(c) 5.3 mol PbS
(d) 5.3 mol O2

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 04:00, josephicarusmarrujo
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1

Chemistry, 23.06.2019 09:00, aaronroberson4940
Weight is a measure of: inertia force matter mass
Answers: 1

Chemistry, 23.06.2019 11:00, randyg0531
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2
Do you know the correct answer?
23. For the reaction shown, calculate how many moles of each
product form when the given amount of...
Questions in other subjects:

Mathematics, 02.09.2020 22:01

History, 02.09.2020 22:01

Mathematics, 02.09.2020 22:01

Chemistry, 02.09.2020 22:01


English, 02.09.2020 22:01

History, 02.09.2020 22:01