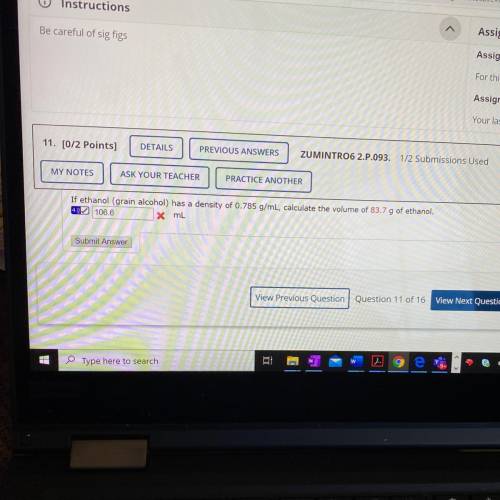
If ethanol has a density of 0.785g/mL, calculate the volume of 83.7 g of ethanol.
...


Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Do you know the correct answer?
Questions in other subjects:

Mathematics, 01.05.2021 14:00


Biology, 01.05.2021 14:00

English, 01.05.2021 14:00


Mathematics, 01.05.2021 14:00

English, 01.05.2021 14:00

English, 01.05.2021 14:10

Mathematics, 01.05.2021 14:10

Mathematics, 01.05.2021 14:10