
Chemistry, 18.10.2020 05:01, cshopholic4921
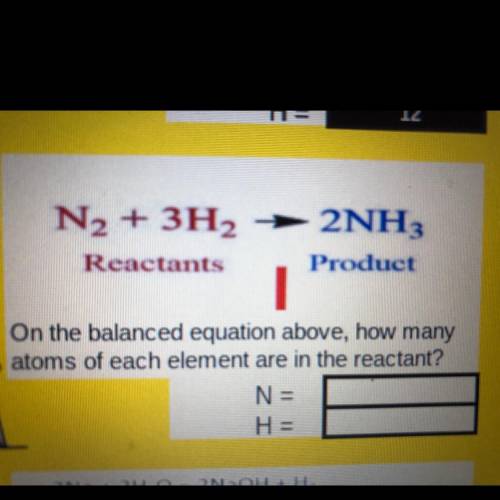
2NH.
N2 + 3H2
Reactants
Product
On the balanced equation above, how many
atoms of each element are in the reactant?
N =
H =

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:30, justinerodriguz2878
What are the major types of a chemical compound
Answers: 2

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
Do you know the correct answer?
2NH.
N2 + 3H2
Reactants
Product
On the balanced equation above, how many
at...
Reactants
Product
On the balanced equation above, how many
at...
Questions in other subjects:

Chemistry, 01.04.2020 06:01







Chemistry, 01.04.2020 06:01


Mathematics, 01.04.2020 06:01






