Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2


Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 23.06.2019 00:30, motorxr714
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
Do you know the correct answer?
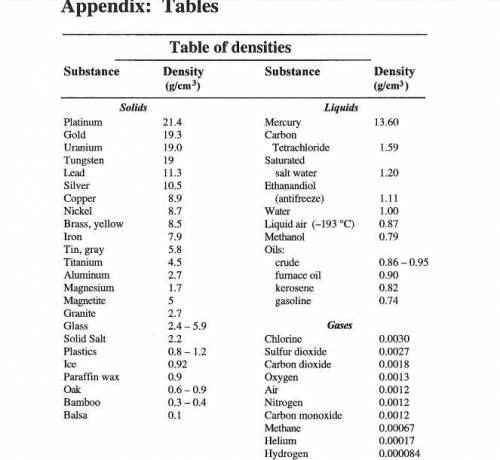
A student measures the mass and volume of a sample of aluminum at room temperature, and calculates t...
Questions in other subjects:

Mathematics, 12.01.2021 20:20

Mathematics, 12.01.2021 20:20

English, 12.01.2021 20:20

Mathematics, 12.01.2021 20:20

Mathematics, 12.01.2021 20:20

English, 12.01.2021 20:20



Mathematics, 12.01.2021 20:20

Mathematics, 12.01.2021 20:20