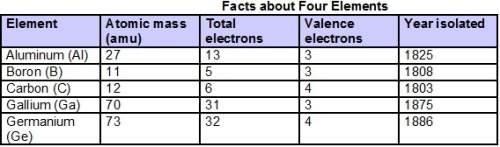
Chemistry, 17.10.2020 06:01, because061907
Aluminum metal reacts with chlorine gas to form aluminum chloride. The reaction can be represented by the following balanced chemical equation:
2 Al(s) + 3 Cl2(g) → 2 AlCl3(s).
If you were trying to react 52.9 grams of chlorine gas completely, how many grams of aluminum would you need?
[grams of Aluminum] Do NOT enter the unit in your answer. Report your answer with 3 SFs.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 00:40, btcastongia
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3


Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1
Do you know the correct answer?
Aluminum metal reacts with chlorine gas to form aluminum chloride. The reaction can be represented b...
Questions in other subjects:


Advanced Placement (AP), 19.02.2021 06:00

Mathematics, 19.02.2021 06:00


Mathematics, 19.02.2021 06:00

Mathematics, 19.02.2021 06:00




Mathematics, 19.02.2021 06:00