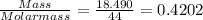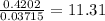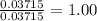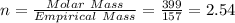Chemistry, 29.08.2019 12:10, cdvazquez727
When a sample of a compound in the vitamin d family was burned in a combustion analysis, 5.983 mg of the compound gave 18.490 mg of co2 and 6.232 mg of h2o. this compound was found to have a molecular mass of 399. what is the molecular formula of this compound? put your answer in form of cxhyoz.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 13:00, torigirl4126
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3
Do you know the correct answer?
When a sample of a compound in the vitamin d family was burned in a combustion analysis, 5.983 mg of...
Questions in other subjects:

Medicine, 26.03.2021 19:10



Mathematics, 26.03.2021 19:10


Mathematics, 26.03.2021 19:10


Mathematics, 26.03.2021 19:10