
Chemistry, 16.10.2020 09:01, 2kdragginppl
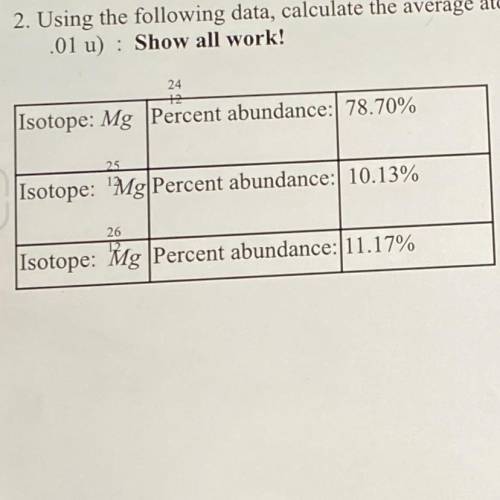
2. Using the following data, calculate the average atomic mass of magnesium (give your answer to the nearest
.01 ) : Show all work!
Isotope: Mg Percent abundance: 78.70%
24
Isotope: 'Mg Percent abundance: 10.13%
25
Isotope:
Mg Percent abundance: 11.17%
26

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 07:00, erickamurillo9929
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 11:20, ashiteru123
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2
Do you know the correct answer?
2. Using the following data, calculate the average atomic mass of magnesium (give your answer to the...
Questions in other subjects:

English, 03.11.2020 17:20





Chemistry, 03.11.2020 17:20









