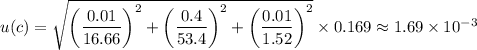Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:00, BrandyLeach01
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
Do you know the correct answer?
you need 16.66ml (+-0.01) of 53.4 (+-0.4)wt% of NaOH with a density of 1.52 (+-0.01)g/mL to prepare...
Questions in other subjects:

English, 03.08.2021 05:40




Medicine, 03.08.2021 05:40




Mathematics, 03.08.2021 05:40