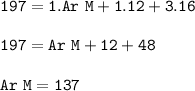Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:10, carmenguabaoql9kv
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium. b)heavier than helium. c)the same weight as helium. d)dependent on the element that reacted with carbon.
Answers: 3

Chemistry, 23.06.2019 00:30, ejuarez2020
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2


Chemistry, 23.06.2019 05:00, jayden6467
How many moles are in 7.2 x 10^23 carbon molecules?
Answers: 1
Do you know the correct answer?
The relative formula mass (M) of a Group 2 metal carbonate is 197
Relative atomic masses (Ar): C =...
Questions in other subjects:


Mathematics, 05.05.2020 03:37







English, 05.05.2020 03:37

Mathematics, 05.05.2020 03:37