

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 07:20, rscvsdfsrysas1857
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1
Do you know the correct answer?
The ph of a 0.25 m aqueous solution of hydrofluoric acid, hf, at 25.0 °c is 2.03. what is the value...
Questions in other subjects:

English, 04.10.2019 18:10


English, 04.10.2019 18:10

Mathematics, 04.10.2019 18:10





Social Studies, 04.10.2019 18:20

Health, 04.10.2019 18:20


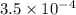
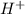 ion.
ion.![pH=-\log [H^+]](/tpl/images/0409/0293/37e81.png)
![2.03=-\log [H^+]](/tpl/images/0409/0293/c70ab.png)
![[H^+]=9.3\times 10^{-3}M](/tpl/images/0409/0293/6b59c.png)
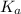 for HF.
for HF.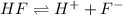
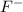 =
= 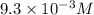
![K_a=\frac{[H^+][F^-]}{[HF]}=\frac{(9.3\times 10^{-3})\times (9.3\times 10^{-3})}{0.25}=3.5\times 10^{-4}](/tpl/images/0409/0293/48d7b.png)




