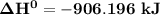Chemistry, 13.10.2020 03:01, giraffesaur44
The first reaction in the Ostwald process for the production of nitric acid involves the combustion of ammonia
4NH3(g)+5O2(g)=4NO(g)+6H2O(g)
a) Estimate ΔH^o (in kJ) for this reaction using average bond energies.
b) Calculate ΔH^o (in kJ) for this reaction using standard heats of formation.
c) Briefly explain why the value for ΔH^o, calculated using average bond energies, is only considered to be an estimate of the standard enthalpy change for the reaction

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, nikkih1225
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1



Chemistry, 22.06.2019 11:10, hannah2757
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1
Do you know the correct answer?
The first reaction in the Ostwald process for the production of nitric acid involves the combustion...
Questions in other subjects:

History, 21.03.2021 17:40

Mathematics, 21.03.2021 17:40


English, 21.03.2021 17:40

Physics, 21.03.2021 17:40

English, 21.03.2021 17:40


Mathematics, 21.03.2021 17:40

Mathematics, 21.03.2021 17:40


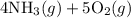 ↔
↔ 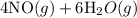
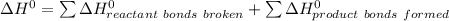
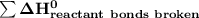 = 7182 kJ
= 7182 kJ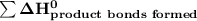 = - 8032 kJ
= - 8032 kJ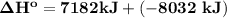

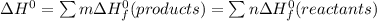
![\Delta H^0 = [ 4 \ mol \times \Delta H^0_f \ (NO(g)) + 6\ mol \times \Delta H^0_f(H_2O)] - [ 4 \ mol \times \Delta H^0_f \ (NH_3(g)) + 5 \ mol \times \Delta H^0_f \ (O_2)]](/tpl/images/0802/3228/2e247.png)
![\Delta H^0 = [ 4 \ mol \times90.29 \ kJ/mol + 6\ mol \times -241.826 \ kJ/mol - [ 4 \ mol \times-45.9 \ kJ/mol + 5 \ mol \times 0]](/tpl/images/0802/3228/35715.png)