
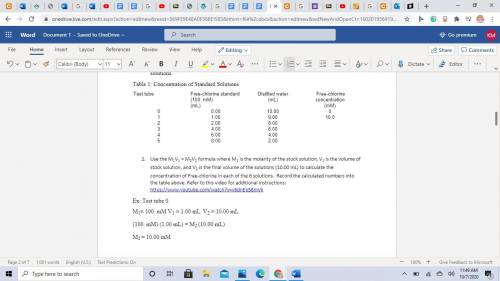
A 100mM/L solution of Free-chlorine stock solution was used to make up 6 standard solutions (known concentration). See the table below with gives the test tube number, the amount of stock solution used and the amount of distilled water used to make up these solutions.
Table 1: Concentration of Standard Solutions
Test tube
Free-chlorine standard (100. mM)
(mL)
Distilled water
(mL)
Free-chlorine concentration
(mM)
0
0.00
10.00
0
1
1.00
9.00
10.0
2
2.00
8.00
3
4.00
6.00
4
6.00
4.00
5
8.00
2.00
Use the M1V1 = M2V2 formula where M1 is the molarity of the stock solution, V1 is the volume of stock solution, and V2 is the final volume of the solutions (10.00 mL) to calculate the concentration of Free-chlorine in each of the 6 solutions. Record the calculated numbers into the table above. Ex: Test tube 0
M1= 100. mM V1 = 1.00 mL V2 = 10.00 mL
(100. mM) (1.00 mL) = M2 (10.00 mL)
M2 = 10.00 mM
(there's also a screenshot of the problem if that's easier)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, drivinghydra
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3
Do you know the correct answer?
A 100mM/L solution of Free-chlorine stock solution was used to make up 6 standard solutions (known c...
Questions in other subjects:

Mathematics, 26.02.2021 02:10

Health, 26.02.2021 02:10







History, 26.02.2021 02:10

Mathematics, 26.02.2021 02:10






