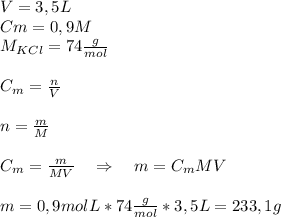How would you prepare 3.5 l of a 0.9m solution of kcl?
a. add 23 g of kcl to a 3.5 l contain...

How would you prepare 3.5 l of a 0.9m solution of kcl?
a. add 23 g of kcl to a 3.5 l container; then add enough water to dissolve the kcl and fill the container to the 3.5 l mark.
b. add 233 g of kcl to a 3.5 l container; then add enough water to dissolve the kcl and fill the container to the 3.5 l mark.
c. add 567 g of kcl to a 3.5 l container; then add enough water to dissolve the kcl and fill the container to the 3.5 l mark.
d. add 287 g of kcl to a 3.5 l container; then add enough water to dissolve the kcl and fill the container to the 3.5 l mark.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 23.06.2019 02:20, alejandraluna95
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1


Do you know the correct answer?
Questions in other subjects:

History, 20.08.2019 20:10

Biology, 20.08.2019 20:10



Mathematics, 20.08.2019 20:10

Mathematics, 20.08.2019 20:10

Engineering, 20.08.2019 20:10

Mathematics, 20.08.2019 20:10