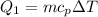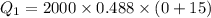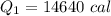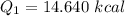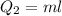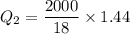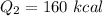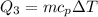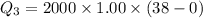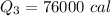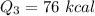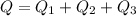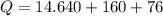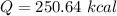Cold-winter survival experts do not recommend eating snow for hydration. One reason for this is the energy it takes to increase temperature of the snow, melt it, and then increase the temperature of the resulting water to body temperature. Calculate the total amount of heat to convert 2.000 kg of snow at -15.00 0C to liquid water at 38.00 0C.
POTENTIALLY HELPFUL INFORMATION:
The heat of fusion for water is 1.44 kcal/mol.
The specific heat capacity of solid water is 0.488 cal/g0C.
The specific heat capacity of liquid water is 1.00 cal/g0C.
The freezing point of water is 00C (also known as melting point).

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chefdnguyen
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 01:30, jusicca1109
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1
Do you know the correct answer?
Cold-winter survival experts do not recommend eating snow for hydration. One reason for this is the...
Questions in other subjects:

Mathematics, 03.03.2021 20:30


Mathematics, 03.03.2021 20:30




Mathematics, 03.03.2021 20:30

Mathematics, 03.03.2021 20:30


Chemistry, 03.03.2021 20:30