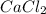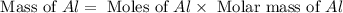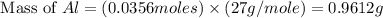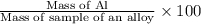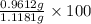5. A 1.1181-g sample of an alloy (a mixture) of aluminum and magnesium was treated with an
excess of sodium hydroxide solution. In the reaction, only the aluminum reacts with the sodium
hydroxide solution:
2 Al + 2 NaOH + 6 H202 Na[Al(OH)4] + 3 H2
If 0.1068 g of H2 is produced, what is the mass percent of aluminum in the alloy?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, jamccoy3335
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1


Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 14:20, kekecantonxox121
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
Do you know the correct answer?
5. A 1.1181-g sample of an alloy (a mixture) of aluminum and magnesium was treated with an
excess o...
Questions in other subjects:

Biology, 30.07.2019 06:00




History, 30.07.2019 06:00



Business, 30.07.2019 06:00

Business, 30.07.2019 06:00


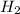 = 0.1068 g
= 0.1068 g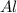 = 27 g/mol
= 27 g/mol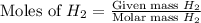
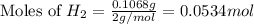
![2Al+2NaOH+6H_2O\rightarrow 2Na[Al(OH)_4]+3H_2](/tpl/images/0796/4861/bae6d.png)
 mole of
mole of