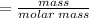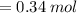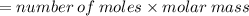Chemistry, 11.10.2020 03:01, cxttiemsp021
How many grams of butane (C H20) must be burned in an excess of O, to produce 15.0 g of CO2? NOTE: Butane reacts with oxygen gas to produce carbon dioxide and water.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, wkalpakchi
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1


Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
Do you know the correct answer?
How many grams of butane (C H20) must be burned in an excess of O, to produce 15.0
g of CO2? NOTE:...
Questions in other subjects:

History, 13.04.2021 23:10

Social Studies, 13.04.2021 23:10




Mathematics, 13.04.2021 23:10


Mathematics, 13.04.2021 23:10

Mathematics, 13.04.2021 23:10