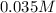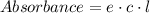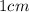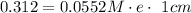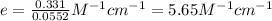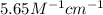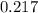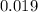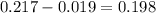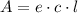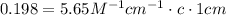Chemistry, 10.10.2020 15:01, GreenHerbz206
Erioglucine is a blue-colored dye that absorbs its complementary color, red, in aqueous solution around 645 nm. Unfortunately, the local distilled water supply used to make solutions is consistently contaminated with a trace amount of a metal cation that also absorbs 645 nm light. Suppose then, a control sample of 0.0552 M erioglucine (aq) has an absorbance of 0.331 and that a distilled water sample in a similar cuvette has an absorbance of 0.019. Determine the concentration of an erioglucine (aq) sample that has an absorbance of 0.217. 10 pts. The answer is .0350 but I wanted to see how to get that answer. You have to subtract .019 from the absorbance of the control and the sample

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, alexabdercmur
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 18:30, robjaykay
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 22.06.2019 21:00, ciel8809
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1
Do you know the correct answer?
Erioglucine is a blue-colored dye that absorbs its complementary color, red, in aqueous solution aro...
Questions in other subjects:

Mathematics, 27.10.2019 16:43



History, 27.10.2019 16:43

English, 27.10.2019 16:43

Mathematics, 27.10.2019 16:43


Mathematics, 27.10.2019 16:43



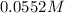 of aqueous solution of erioglaucine has absorbance of
of aqueous solution of erioglaucine has absorbance of