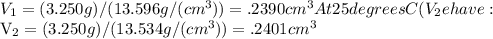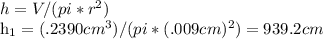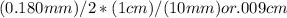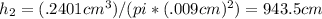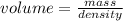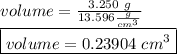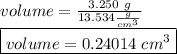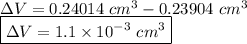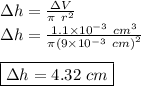Chemistry, 04.10.2019 18:30, treyvoniskewl
Suppose a mercury thermometer contains 3.250g of mercury and has a capillary that is 0.180mm in diameter.. how far does the mercury rise in the capillary when the temperature changes from 0.0 ∘c to 25.0 ∘c? the density of mercury at these temperatures is 13.596 g/cm3 and 13.534 g/cm3, respectively.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1


Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 23.06.2019 08:00, Robinlynn228
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1
Do you know the correct answer?
Suppose a mercury thermometer contains 3.250g of mercury and has a capillary that is 0.180mm in diam...
Questions in other subjects:

English, 08.07.2019 15:50

English, 08.07.2019 15:50



Advanced Placement (AP), 08.07.2019 15:50

Mathematics, 08.07.2019 15:50

Mathematics, 08.07.2019 15:50

Mathematics, 08.07.2019 15:50

Mathematics, 08.07.2019 15:50

English, 08.07.2019 15:50