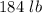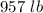Chemistry, 08.10.2020 19:01, amiechap12
7 Salt water containing 1.92 lb/gal of salt flows at a fixed rate of 2 gal/min into a 100 gal tank, initially filled with fresh water. The density of the incoming solution is 71.8 lb/ft3. The solution, kept uniform by stirring, flows out at a fixed rate of 19.2 lb/min. How many pounds of salt will there be in the tank at the end of 1 h and 40 min? What is the upper limit for the number of pounds of salt in the tank if the process continues indefinitely? How much time will elapse while the quantity of salt in the tank changes from 100 to 150 lb?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, amariyanumber1923
19. at high pressures, how does the volume of a real gas compare with the volume of an ideal gas under the same conditions, and why? eman- it is much less because real gas partides are not moving. there is no difference because the gas laws are always obeyed. it is much less because at high pressures the temperature drops. it is much greater because real gas partides take up space.
Answers: 1


Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 23.06.2019 00:30, clairebear66
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
Do you know the correct answer?
7 Salt water containing 1.92 lb/gal of salt flows at a fixed rate of 2 gal/min into a 100 gal tank,...
Questions in other subjects:


Mathematics, 31.05.2021 20:30





Social Studies, 31.05.2021 20:30




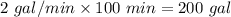
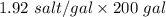
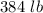
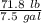
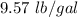
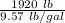
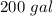 (salt went out)
(salt went out)