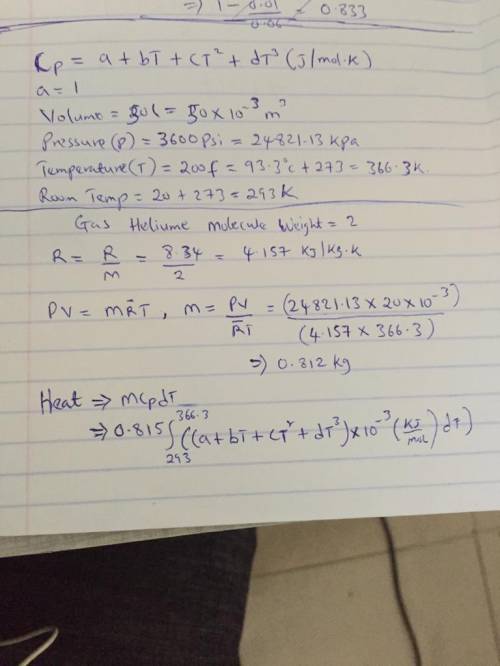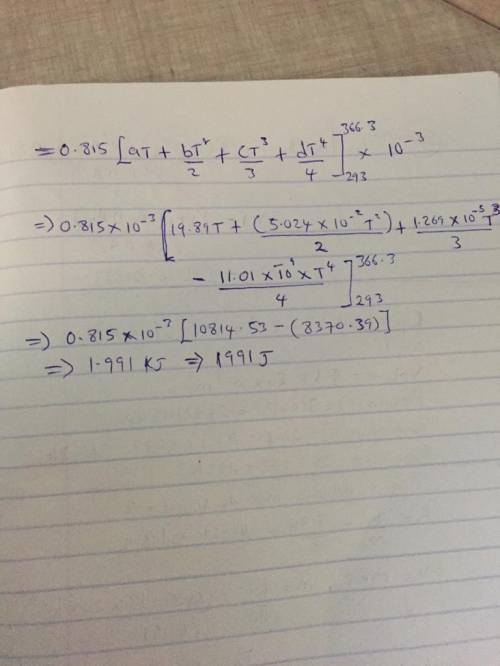The specific heats at constant pressure of some common gases are provided as a thirdorder polynomial: �;<<< = � + �� + ��C + ��E, with units J/(mol K). For methane (CH4) the coefficients are � = 19.89, � = 5.024 × 10NC, � = 1.269 × 10NO, � = −11.01 × 10NQ. At a production facility, the gas in a 50-liter tank is compressed to 3,600 psi (gage) during which time the temperature rises to 200°F. How much heat in J is given off as the gas cools to room temperature? Suppose the compressed gas is helium, how much heat would be given off in this case?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, ayoismeisalex
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 14:30, joejoefofana
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀ pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4. 0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3
Do you know the correct answer?
The specific heats at constant pressure of some common gases are provided as a thirdorder polynomial...
Questions in other subjects:

Mathematics, 23.04.2020 16:55

Biology, 23.04.2020 16:55






Mathematics, 23.04.2020 16:55

Mathematics, 23.04.2020 16:55