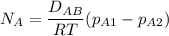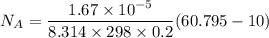The gas CO2 is diffusing at steady state through a tube 0.20 meters long. The tube has a diameter of 0.01 meters and also contains N2 at 298 K. The total pressure inside the tube is constant at 101.32 kPa. The partial pressure of CO2 is 456 mm Hg at one end and 76 mm Hg at the other end. The diffusion coefficient of CO2 in N2 is 1.67 x 10-5 m2 /sec at 298 K. Calculate the molar flux of CO2 in SI units, assuming equimolar counter-diffusion between the CO2 and N2 gases.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, Johnson926
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

Chemistry, 23.06.2019 05:30, xarianna2007
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1
Do you know the correct answer?
The gas CO2 is diffusing at steady state through a tube 0.20 meters long. The tube has a diameter of...
Questions in other subjects:



Mathematics, 19.11.2019 17:31





Mathematics, 19.11.2019 17:31

English, 19.11.2019 17:31


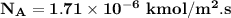
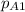 is 456 mm Hg at one end
is 456 mm Hg at one end 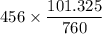
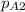 at the other end is 76 mm Hg
at the other end is 76 mm Hg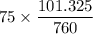
 10 kPa
10 kPa in N
in N