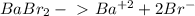Chemistry, 15.10.2019 04:30, DEVORIA2001
Determine the concentrations of babr2, ba2 , and br– in a solution prepared by dissolving 2.37 × 10–4 g babr2 in 1.00 l of water. express all three concentrations in molarity. additionally, express the concentrations of the ionic species in parts per million (ppm).

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, kathleensumter4913
219 grams of iron (iii) oxide reacts with excess carbon according to the reaction equation shown below. fe2o3 + c → fe + co2 after a scientist performs the chemical reaction they find the actual yield of iron to be 57.4 grams. calculate the percent yield of this chemical reaction.
Answers: 1



Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
Do you know the correct answer?
Determine the concentrations of babr2, ba2 , and br– in a solution prepared by dissolving 2.37 × 10–...
Questions in other subjects:

Mathematics, 15.12.2020 21:40

Mathematics, 15.12.2020 21:40

Mathematics, 15.12.2020 21:40

Mathematics, 15.12.2020 21:40

Mathematics, 15.12.2020 21:40


Mathematics, 15.12.2020 21:40


Mathematics, 15.12.2020 21:40