Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, tgraveslaylay2743
Bose-einstein condensation occurs at what temperature?
Answers: 2


Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
Do you know the correct answer?
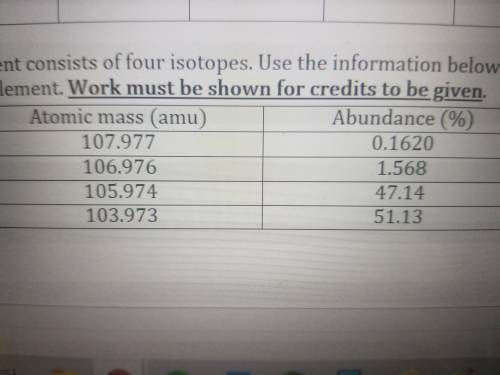
A hypothetical element consists of four isotopes. Use the information below to calculate the average...
Questions in other subjects:


Mathematics, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30