
Chemistry, 06.10.2020 17:01, Jsquad8879
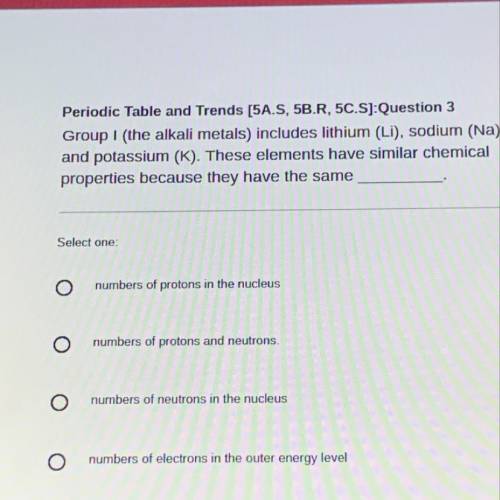
Group I (the alkali metals) includes lithium (LI), sodium (Na),
and potassium (K). These elements have similar chemical
properties because they have the same

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:40, Wemaybewrong
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
Do you know the correct answer?
Group I (the alkali metals) includes lithium (LI), sodium (Na),
and potassium (K). These elements h...
Questions in other subjects:

Mathematics, 16.12.2020 02:30






Biology, 16.12.2020 02:30

Biology, 16.12.2020 02:30

Mathematics, 16.12.2020 02:30

Spanish, 16.12.2020 02:30






