
Chemistry, 05.10.2020 14:01, zahradawkins2007
The average atomic mass of molybdenum (Mo) is 95.94 amu. Which of the following isotopes must have the highest percent abundance?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Kaylinne1181
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 21.06.2019 21:00, alexmarche4675
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 21.06.2019 22:00, toledanomariap43bxm
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 00:30, xoxokaydavis5837
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1
Do you know the correct answer?
The average atomic mass of molybdenum (Mo) is 95.94 amu. Which of the following isotopes must have t...
Questions in other subjects:



Mathematics, 07.06.2021 16:40


Mathematics, 07.06.2021 16:40




Mathematics, 07.06.2021 16:40


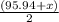 = 95.95 amu
= 95.95 amu



