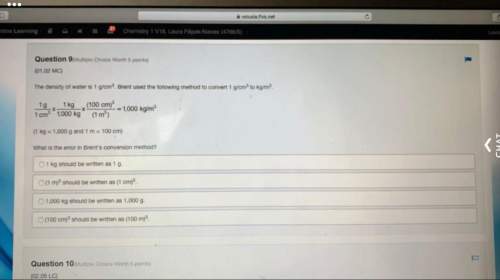
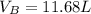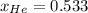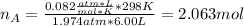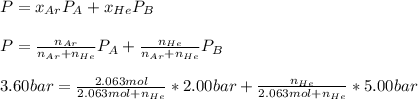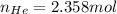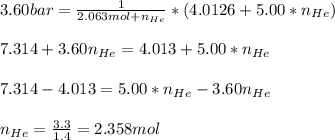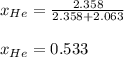Chemistry, 04.10.2020 17:01, devenybates
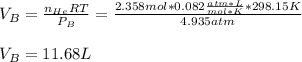
Argon (Ar) and helium (He) are initially in separate compartments of a container at 25°C. The
Ar in compartment A, which has a volume VA of 6.00 L, has a pressure of 2.00 bar. The He in
compartment B of unknown volume V3 has a pressure of 5.00 bar. When the two compartments
are connected and the gases allowed to mix, the total pressure of gas is 3.60 bar. Assume both
gases behave ideally
(a) [4 marks) Determine the volume of compartment B.
(b) [2 marks] Determine the mole fraction of He in the mixture of gases.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
Do you know the correct answer?
Argon (Ar) and helium (He) are initially in separate compartments of a container at 25°C. The
Ar in...
Questions in other subjects:




English, 16.10.2020 06:01


Mathematics, 16.10.2020 06:01