
Chemistry, 02.10.2020 19:01, jtswagg6634
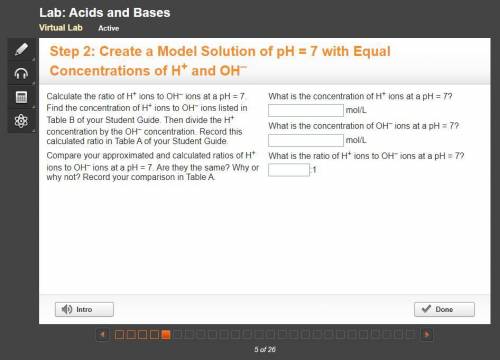
10 PTS FOR CORRECT ANSWER Calculate the ratio of H+ ions to OH– ions at a pH = 7. Find the concentration of H+ ions to OH– ions listed in Table B of your Student Guide. Then divide the H+ concentration by the OH– concentration. Record this calculated ratio in Table A of your Student Guide.
Compare your approximated and calculated ratios of H+ ions to OH– ions at a pH = 7. Are they the same? Why or why not? Record your comparison in Table A.
What is the concentration of H+ ions at a pH = 7?
mol/L
What is the concentration of OH– ions at a pH = 7?
mol/L
What is the ratio of H+ ions to OH– ions at a pH = 7?
:1

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2


Chemistry, 22.06.2019 13:30, citlalli30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
Do you know the correct answer?
10 PTS FOR CORRECT ANSWER Calculate the ratio of H+ ions to OH– ions at a pH = 7. Find the concentra...
Questions in other subjects:

Geography, 13.08.2021 14:00

Mathematics, 13.08.2021 14:00

History, 13.08.2021 14:00

Biology, 13.08.2021 14:00

Health, 13.08.2021 14:00


History, 13.08.2021 14:00

Mathematics, 13.08.2021 14:00

Social Studies, 13.08.2021 14:00

Business, 13.08.2021 14:00






