
Chemistry, 02.10.2020 16:01, ashvinmsingh
calculate the heat required to melt 25.7g of solid methanol (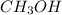 at its melting point ( Hfus=3.22KJ/mol , Mm=32g/mol)
at its melting point ( Hfus=3.22KJ/mol , Mm=32g/mol)


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, mimireds5419
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 01:40, janelisse199820
Non renewable resources like petroleum eventually
Answers: 2

Chemistry, 22.06.2019 13:00, carlinryan
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 15:00, makaylajones74pdxtrk
What is the most important factor in determining climates.
Answers: 1
Do you know the correct answer?
calculate the heat required to melt 25.7g of solid methanol ( at its melting point ( Hfus=3.22KJ/mol...
Questions in other subjects:


History, 21.10.2020 19:01



Social Studies, 21.10.2020 19:01

Mathematics, 21.10.2020 19:01

English, 21.10.2020 19:01


Mathematics, 21.10.2020 19:01

Computers and Technology, 21.10.2020 19:01






