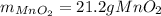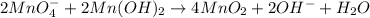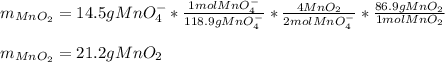Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:10, hannah2757
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1


Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 22:30, StupidFatChipmunk
What must be in balance for temperatures to remain constant?
Answers: 1
Do you know the correct answer?
If 14.5 g of MnO4- (permanganate) react with manganese (II) hydroxide how many grams of manganese (I...
Questions in other subjects:


Mathematics, 18.10.2020 06:01

Social Studies, 18.10.2020 06:01

Chemistry, 18.10.2020 06:01

History, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01

Arts, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01