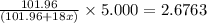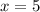Chemistry, 25.09.2020 06:01, witchhunt666
A sample of pure alumina hydrate was obtained. A 5.000 g sample of the material was heated carefully in a vacuum oven until no more mass was lost from the sample. After heating, the final weight of the material was 2.6763 g. What was the formula of the hydrated alumina, Al2O3•xH2O? (Enter a whole number for "x") (mol. wt. Al2O3 = 101.96)

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 11:30, nadine6085859r
Which of the following is a property of nonmetals? a. nonmetals are ductile. b. nonmetals have a shiny luster. c. nonmetals have high density. d. nonmetals are nonconductors.
Answers: 1

Chemistry, 23.06.2019 15:40, thatlostgirl7740
Which functions of water in living systems would still be possible if water was not polar and did not form hydrogen bonds? check all that apply. climate regulation dissolving ionic compounds for biological reactions providing body support by exerting pressure on cell walls providing body support through buoyancy transport of nutrients within organisms temperature regulation in many organisms
Answers: 3

Chemistry, 23.06.2019 16:20, chimwim8347
Select the correct answer. what is the wavelength of radio waves? a. between 1 x 10-3 and 1 x 10-1 meters b. less than 1 x 10-11 meters c. more than 1 x 10-1 meters d. between 1 x 10-8 - 4 x 10-7 meters e. between 4 x 10-7 - 7 x 10-7 meters
Answers: 3
Do you know the correct answer?
A sample of pure alumina hydrate was obtained. A 5.000 g sample of the material was heated carefully...
Questions in other subjects:










Biology, 12.07.2019 18:00


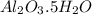
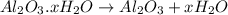
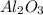 = 101.96 g/mol
= 101.96 g/mol
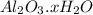 decomposes to give 101.96 g of
decomposes to give 101.96 g of 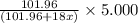 of H_2O
of H_2O