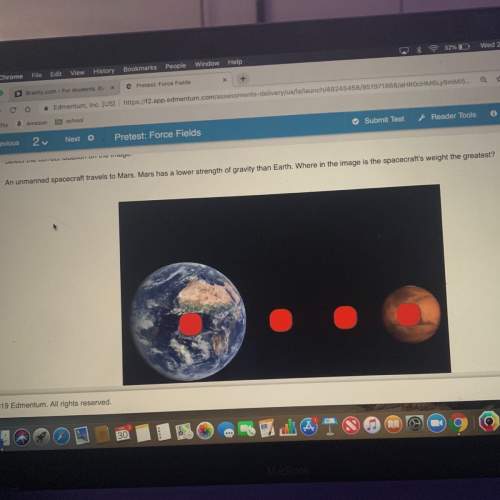
HELP! A typical weather balloon contains approximately 563 L of hydrogen gas when it is released. If the temperature and pressure at the surface are 288 K and 1.00 atm when the balloon is released, what will its volume be at 32.0 km above the surface, where temperature and pressure are 299 K and 8.57 x 10^-3 atm?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
Do you know the correct answer?
HELP! A typical weather balloon contains approximately 563 L of hydrogen gas when it is released. If...
Questions in other subjects:





Biology, 05.05.2020 21:35

Mathematics, 05.05.2020 21:35