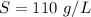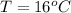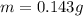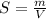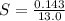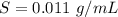Chemistry, 22.09.2020 21:01, ncontreras06
A geochemist in the field takes a 13.0 mL sample of water from a rock pool lined with crystals of a certain mineral compound X. He notes the temperature of the pool, 16.° C, and caps the sample carefully. Back in the lab, the geochemist filters the sample and then evaporates all the water under vacuum. Crystals of X are left behind. The researcher washes, dries and weighs the crystals. They weigh 0.143 g
Required:
Using only the information above, can you calculate the solubility of X in water at 15°C ? If you said yes, calculate it.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
Do you know the correct answer?
A geochemist in the field takes a 13.0 mL sample of water from a rock pool lined with crystals of a...
Questions in other subjects:


Mathematics, 20.09.2019 12:20



History, 20.09.2019 12:20

Mathematics, 20.09.2019 12:20


Chemistry, 20.09.2019 12:20

Geography, 20.09.2019 12:20

English, 20.09.2019 12:20