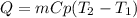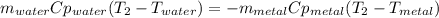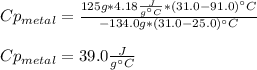Chemistry, 22.09.2020 04:01, jakiyahporter0817
A 134.0 g sample of an unknown metal is heated to 91.0⁰C and then placed in 125 g of water at 25.0⁰C. The final temperature of the water is measured at 31.0⁰C. Calculate the specific heat capacity of the unknown metal. Specific Heat of water is 4.18 J/g*C pls answer as quickly as possible

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3


Chemistry, 22.06.2019 14:30, belindajolete
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
Do you know the correct answer?
A 134.0 g sample of an unknown metal is heated to 91.0⁰C and then placed in 125 g of water at 25.0⁰C...
Questions in other subjects:


Mathematics, 25.02.2021 04:10


History, 25.02.2021 04:10

Mathematics, 25.02.2021 04:10

Biology, 25.02.2021 04:10



English, 25.02.2021 04:10