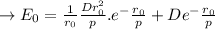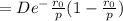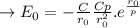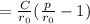Chemistry, 21.09.2020 09:01, mariaveliz2201
The net potential energy EN between two adjacent ions, is sometimes represented by the expression
EN = -(C/r) + D exp(-r/rho)
in which r is the interionic separation and C, D, and rho are constants whose values depend on the specific material.
Required:
a. Derive an expression for the bonding energy E0 in terms of the equilibrium interionic separation r0 and the constants D and rho using the following procedure:
1. Differentiate EN with the respect to r and set the resulting expression equal to zero.
2. Solve for C in terms of D, rho, and ro.
3. Determine the expression for Eo by substitution for C in the equation EN = -(C/r) + D exp(-r/rho)
b. Derive another expression for Eo in terms of ro, C, and rho using a procedure analogous to the one outlined in part (a).

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:30, liyahlanderson2232
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 01:00, kwarwick0915
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Do you know the correct answer?
The net potential energy EN between two adjacent ions, is sometimes represented by the expression
E...
Questions in other subjects:


Mathematics, 07.07.2019 06:00

Spanish, 07.07.2019 06:00




Mathematics, 07.07.2019 06:00

Mathematics, 07.07.2019 06:00

Social Studies, 07.07.2019 06:00

Mathematics, 07.07.2019 06:00


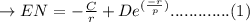
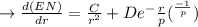
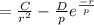
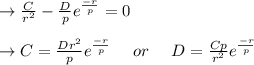
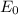 value is replaced by the C value in (1):
value is replaced by the C value in (1):