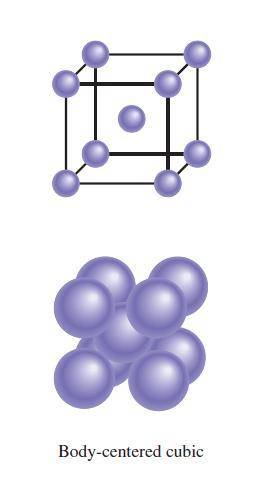
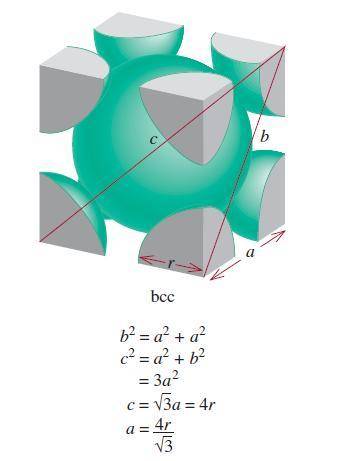
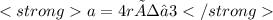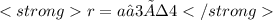A certain metal crystallizes in a lattice described by a body-centered cubic (bcc) unit cell. The lattice constant has been measured by X-ray crystallography to be . Calculate the radius of an atom of . Be sure your answer has significant digits, and be sure it has the correct unit symbol.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, minstcordell4115
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 23.06.2019 02:20, theactualslash
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2


Chemistry, 23.06.2019 10:30, GiuliAzevedo
The element chlorine has two stable isotopes, chlorine-35 with a mass of 34.97 amu and chlorine-37 with a mass of 36.95 amu. from the atomic weight of cl = 35.45 one can conclude that:
Answers: 2
Do you know the correct answer?
A certain metal crystallizes in a lattice described by a body-centered cubic (bcc) unit cell. The la...
Questions in other subjects:

Mathematics, 05.05.2020 00:59


Chemistry, 05.05.2020 00:59



Mathematics, 05.05.2020 00:59

Biology, 05.05.2020 00:59

Mathematics, 05.05.2020 00:59

Mathematics, 05.05.2020 00:59

English, 05.05.2020 00:59