Chemistry, 20.09.2020 14:01, swkgp3cevk
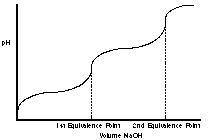
When a diprotic acid is titrated with a strong base, and the Ka1 and Ka2 are significantly different, then the pH vs. volume plot of the titration will have Group of answer choices

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, tifftifftiff5069
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1


Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 23:00, Kykebailey2356
Which of two curves exhibits exponential growth
Answers: 1
Do you know the correct answer?
When a diprotic acid is titrated with a strong base, and the Ka1 and Ka2 are significantly different...
Questions in other subjects:


Physics, 08.08.2019 22:20






Mathematics, 08.08.2019 22:20

Mathematics, 08.08.2019 22:20