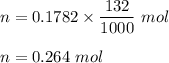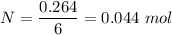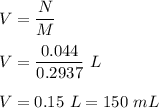Chemistry, 20.09.2020 15:01, genesiloves
Iron in the 2 oxidation state reacts with potassium dichromate to produce Fe3 and Cr3 according to the equation: 6 Fe2 (aq) Cr2O72-(aq) 14 H (aq) <> 6 Fe3 (aq) 2 Cr3 (aq) 7 H2O(l) How many milliliters of 0.2937 M K2Cr2O7 are required to titrate 132.0 mL of 0.1782 M Fe2 solution

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 19:30, Karinaccccc
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
Do you know the correct answer?
Iron in the 2 oxidation state reacts with potassium dichromate to produce Fe3 and Cr3 according to t...
Questions in other subjects:

Computers and Technology, 30.11.2021 01:20




SAT, 30.11.2021 01:20

SAT, 30.11.2021 01:20





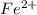 , V = 132 mL .
, V = 132 mL .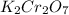 are required to titrate 132.0 mL of 0.1782 M
are required to titrate 132.0 mL of 0.1782 M 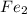 :
: