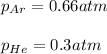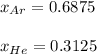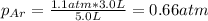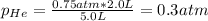Chemistry, 20.09.2020 15:01, noeliaortiz3478
Two containers, one with a volume of 3.0 L and the other with a volume of 2.0 L contain, respectively, argon gas at 1.1 atm and helium at 0.75 atm. The containers are initially separated by a valve, and then the valve is opened to connect the two containers. Assume perfect gases and determine the followings.
a. The total pressure of the mixed gases
b. The partial pressure of each gas
c. The mole fraction of each gas

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, maddyjones4172
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3


Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
Do you know the correct answer?
Two containers, one with a volume of 3.0 L and the other with a volume of 2.0 L contain, respectivel...
Questions in other subjects:

Health, 15.10.2019 12:30

Chemistry, 15.10.2019 12:30

Arts, 15.10.2019 12:30

Business, 15.10.2019 12:30

Health, 15.10.2019 12:30

Mathematics, 15.10.2019 12:30


Mathematics, 15.10.2019 12:30

Mathematics, 15.10.2019 12:30

Geography, 15.10.2019 12:30


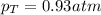 .
.