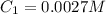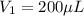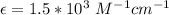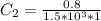Chemistry, 20.09.2020 17:01, haydenamrhein1693
In a laboratory experiment, you are asked to determine the molar concentration of a solution of an unknown compound, X. The solution diluted in with water (200 µL of X + 800 µL of H2O) has an absorbance at 425 nm of 0.8 and a molar extinction coefficient of 1.5 x103 M-1cm-1 at 425 nm. What is the molar concentration of the original solution of X?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3


Chemistry, 22.06.2019 21:00, thebasedgodchri
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1
Do you know the correct answer?
In a laboratory experiment, you are asked to determine the molar concentration of a solution of an u...
Questions in other subjects:







Social Studies, 28.01.2020 02:31

History, 28.01.2020 02:31

Computers and Technology, 28.01.2020 02:31