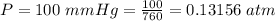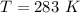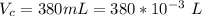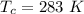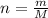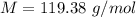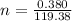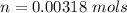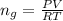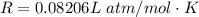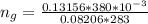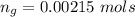Chemistry, 20.09.2020 18:01, juanitarodrigue
The vapor pressure of liquid chloroform, CHCl3, is 100. mm Hg at 283 K. A 0.380 g sample of liquid CHCl3 is placed in a closed, evacuated 380. mL container at a temperature of 283 K.
Assuming that the temperature remains constant, will all of the liquid evaporate? yes/no
What will the pressure in the container be when equilibrium is reached? mm Hg

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, xoxokaydavis5837
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 21:20, skyemichellec
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
Do you know the correct answer?
The vapor pressure of liquid chloroform, CHCl3, is 100. mm Hg at 283 K. A 0.380 g sample of liquid C...
Questions in other subjects:

Law, 21.06.2021 14:00

Mathematics, 21.06.2021 14:00


Mathematics, 21.06.2021 14:00


Health, 21.06.2021 14:00

Business, 21.06.2021 14:00


English, 21.06.2021 14:00