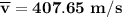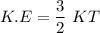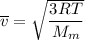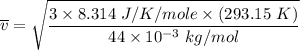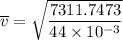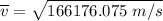Consider a closed container of gas that is a mixture of 30% CO2 and 70% N2 at room temperature 20°C. The gases are in thermal equilibrium with one another. a) Which has the higher kinetic energy, the average CO2 or N2 molecule? b) What is that root-mean-square velocity of a CO2 molecule? For reference, a carbon atom has 6 protons, a nitrogen atom has 7 protons, and an oxygen atom has 8 protons.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 07:00, misspicafunpoke
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Do you know the correct answer?
Consider a closed container of gas that is a mixture of 30% CO2 and 70% N2 at room temperature 20°C....
Questions in other subjects:

Mathematics, 19.10.2020 21:01

Chemistry, 19.10.2020 21:01

Mathematics, 19.10.2020 21:01