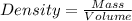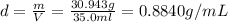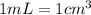Chemistry, 19.12.2019 16:31, jaydenromero31
If 30.943 g of a liquid occupy a space of 35.0 ml. what is the density of the liquid in g/cm3?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1

Chemistry, 22.06.2019 22:30, xlebrny7831
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 02:20, theactualslash
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
Do you know the correct answer?
If 30.943 g of a liquid occupy a space of 35.0 ml. what is the density of the liquid in g/cm3?...
Questions in other subjects:

Mathematics, 04.11.2020 20:40

Chemistry, 04.11.2020 20:40

English, 04.11.2020 20:40


Mathematics, 04.11.2020 20:40


Mathematics, 04.11.2020 20:40


Mathematics, 04.11.2020 20:40


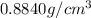 is the density of the liquid.
is the density of the liquid.