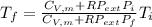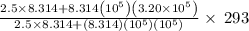An automobile tire contains air at 320.×103 Pa at 20.0 ◦C. The stem valve is removed and the air is allowed to expand adiabatically against the constant external pressure of 100.×103 Pa until P = Pexternal. Assume the air is an ideal gas with C¯ V = 5/2 R (diatomic). Calculate the final temperature.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, taralynnn8870
Fugu, also known as puffer fish, is a sushi delicacy that can also be lethal. puffer fish contain a powerful toxin that can kill an adult a few hours after ingestion. sushi chefs who prepare fugu must be specially trained because any contamination of the toxin-free areas of the fish can be deadly. recently this toxin has been put to good use, as scientists have discovered that a purified form of it can treat severe pain in cancer patients. this recent scientific discovery would fall under which area of chemistry? applied biochemistry pure organic chemistry pure physical chemistry applied inorganic chemistry
Answers: 1

Chemistry, 22.06.2019 15:00, makaylajones74pdxtrk
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 23.06.2019 00:50, lakhanir2013
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
Do you know the correct answer?
An automobile tire contains air at 320.×103 Pa at 20.0 ◦C. The stem valve is removed and the air is...
Questions in other subjects:


Mathematics, 30.09.2019 17:10

Biology, 30.09.2019 17:10



Business, 30.09.2019 17:10

Physics, 30.09.2019 17:10

Social Studies, 30.09.2019 17:10